INDICATION & IMPORTANT SAFETY INFORMATION
INDICATION
IMPORTANT SAFETY INFORMATION
INDICATION
ZEJULA (niraparib) is indicated:
- for first-line maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy and whose cancer is associated with homologous recombination deficiency (HRD) - positive status defined by either a deleterious or suspected deleterious BRCA mutation, and/or genomic instability.
IMPORTANT SAFETY INFORMATION
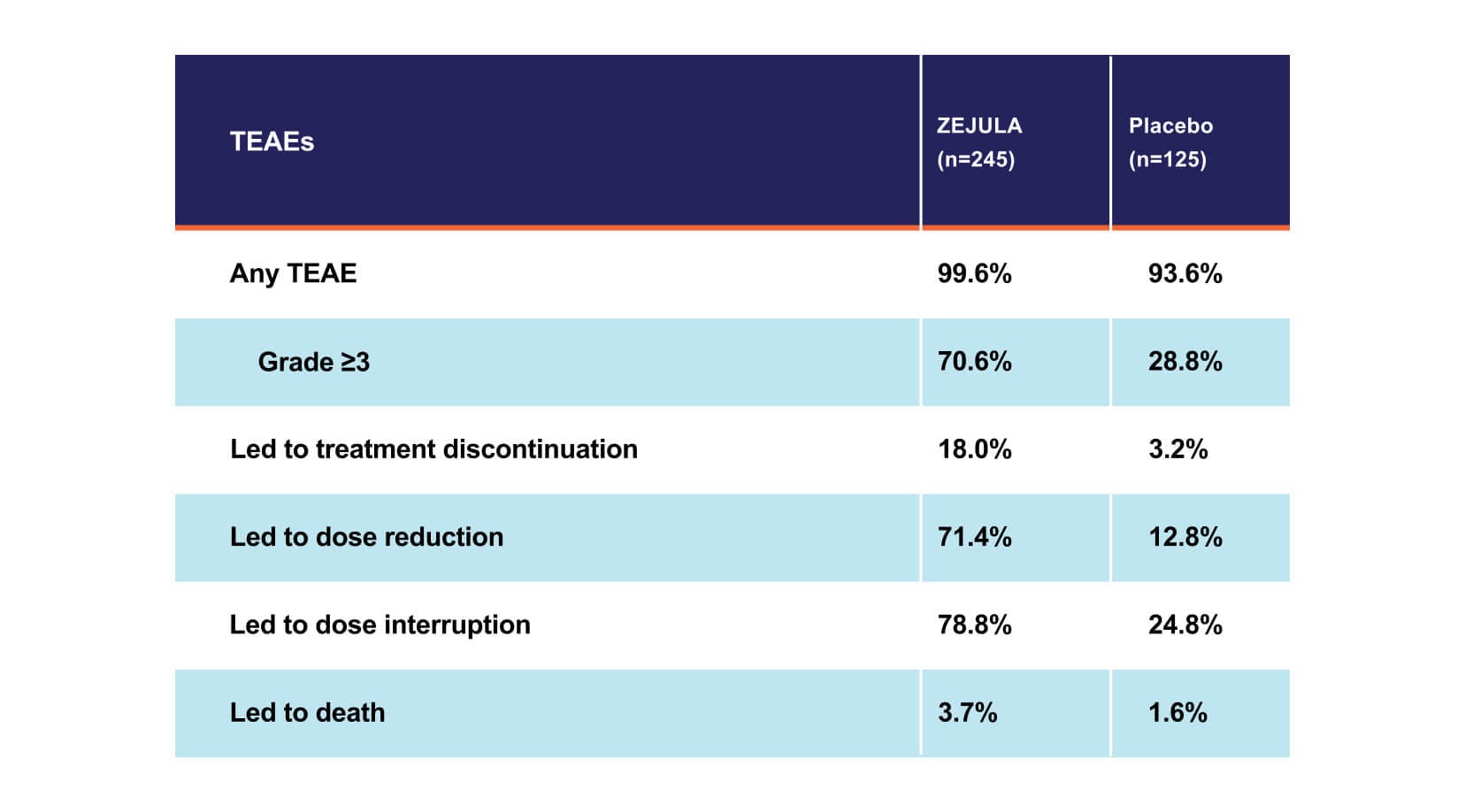
Myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), including cases with a fatal outcome, have been reported in patients who received ZEJULA. In PRIMA, of patients within the HRD-positive population, MDS/AML occurred in 8 out of 245 (3.3%) patients treated with ZEJULA, and in 3 out of 125 (2.4%) patients treated with placebo with a follow-up of 6.1 years. The duration of therapy with ZEJULA in patients who developed secondary MDS/cancer therapy-related AML varied from 5.5 months to 5 years. All patients who developed secondary MDS/cancer therapy-related AML had received previous chemotherapy with platinum agents and/or other DNA-damaging agents, including radiotherapy. For suspected MDS/AML or prolonged hematological toxicities, refer the patient to a hematologist for further evaluation. Discontinue ZEJULA if MDS/AML is confirmed.
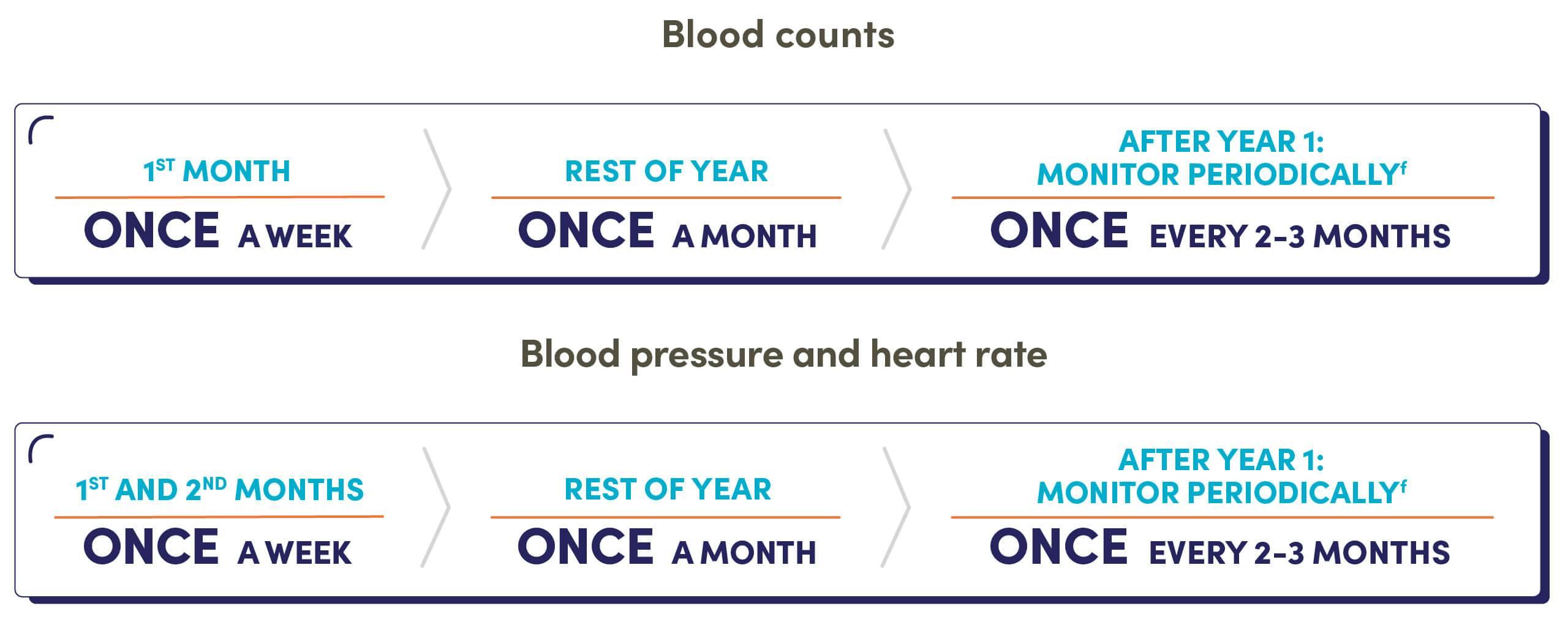
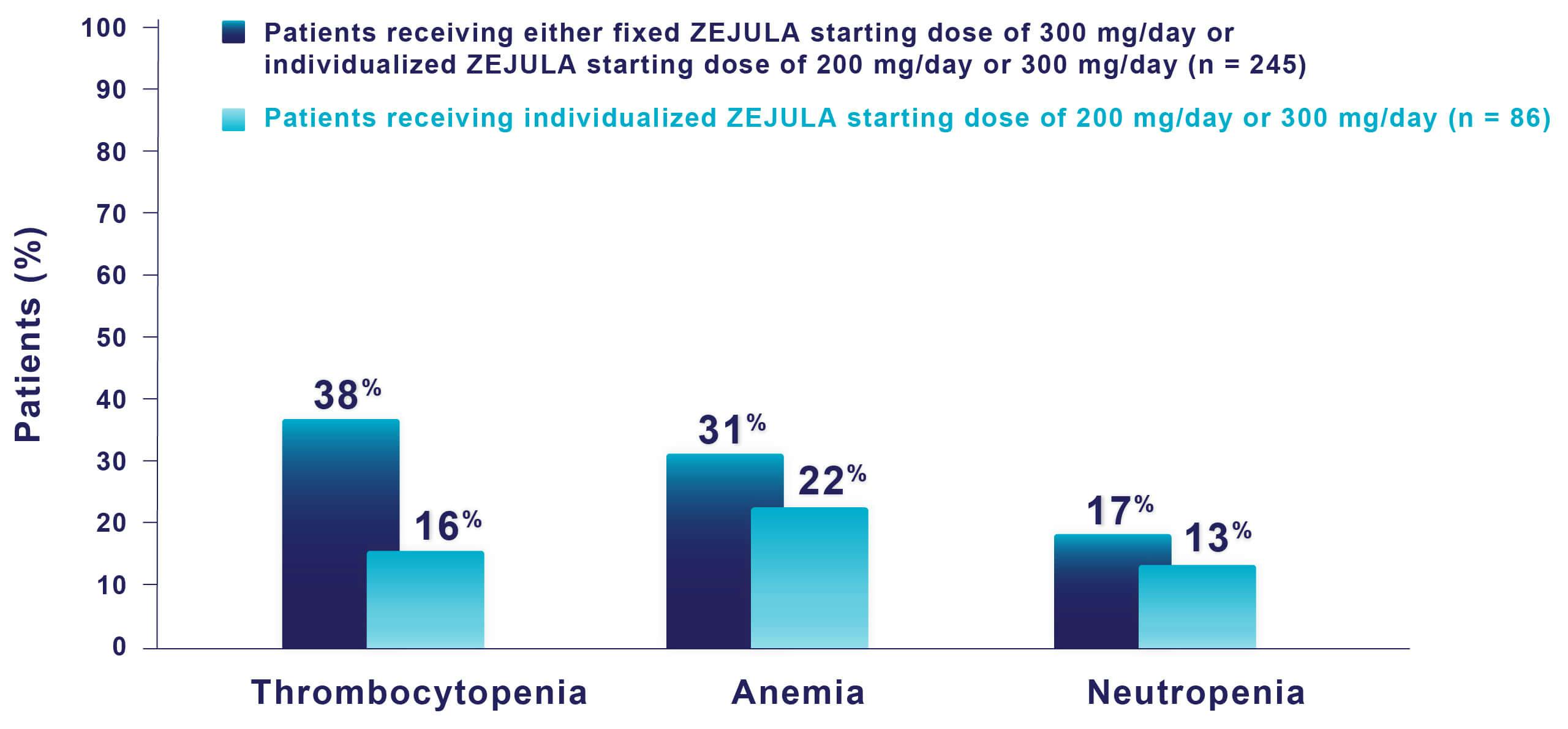
Hematologic adverse reactions (thrombocytopenia, anemia, neutropenia, and/or pancytopenia) have been reported in patients receiving ZEJULA. The overall incidence of Grade ≥3 thrombocytopenia, anemia, and neutropenia were reported, respectively, in 39%, 31%, and 21% of patients receiving ZEJULA in PRIMA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 4%, 2%, and 2% of patients in PRIMA. In patients who were administered a starting dose of ZEJULA based on baseline weight or platelet count in PRIMA, Grade ≥3 thrombocytopenia, anemia, and neutropenia were reported, respectively, in 22%, 23%, and 15% of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred, respectively, in 3%, 3%, and 2% of patients. Do not start ZEJULA until patients have recovered from hematological toxicity caused by prior chemotherapy (≤Grade 1). Monitor complete blood counts weekly for the first month, monthly for the next 11 months, and periodically thereafter. If hematological toxicities do not resolve within 28 days following interruption, discontinue ZEJULA, and refer the patient to a hematologist for further investigations.
Hypertension and cardiovascular effects have been reported in patients receiving ZEJULA. Grade 3-4 hypertension occurred in 6% of patients receiving ZEJULA vs 1% of patients receiving placebo in PRIMA, with no reported discontinuations. Monitor blood pressure and heart rate at least weekly for the first two months, then monthly for the first year, and periodically thereafter during treatment with ZEJULA. Closely monitor patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Manage hypertension with antihypertensive medications and adjustment of the ZEJULA dose if necessary.
Posterior reversible encephalopathy syndrome (PRES) occurred in 0.1% of 2,165 patients treated with ZEJULA in clinical trials and has also been described in postmarketing reports. Monitor all patients for signs and symptoms of PRES, which include seizure, headache, altered mental status, visual disturbance, or cortical blindness, with or without associated hypertension. Diagnosis requires confirmation by brain imaging. If suspected, promptly discontinue ZEJULA and administer appropriate treatment. The safety of reinitiating ZEJULA is unknown.
Embryo-fetal toxicity and lactation: Based on its mechanism of action, ZEJULA can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment and for 6 months after receiving their final dose of ZEJULA. Because of the potential for serious adverse reactions from ZEJULA in breastfed infants, advise lactating women not to breastfeed during treatment with ZEJULA and for 1 month after receiving the last dose.
First-line Maintenance Treatment of HRD-Positive Advanced Ovarian Cancer
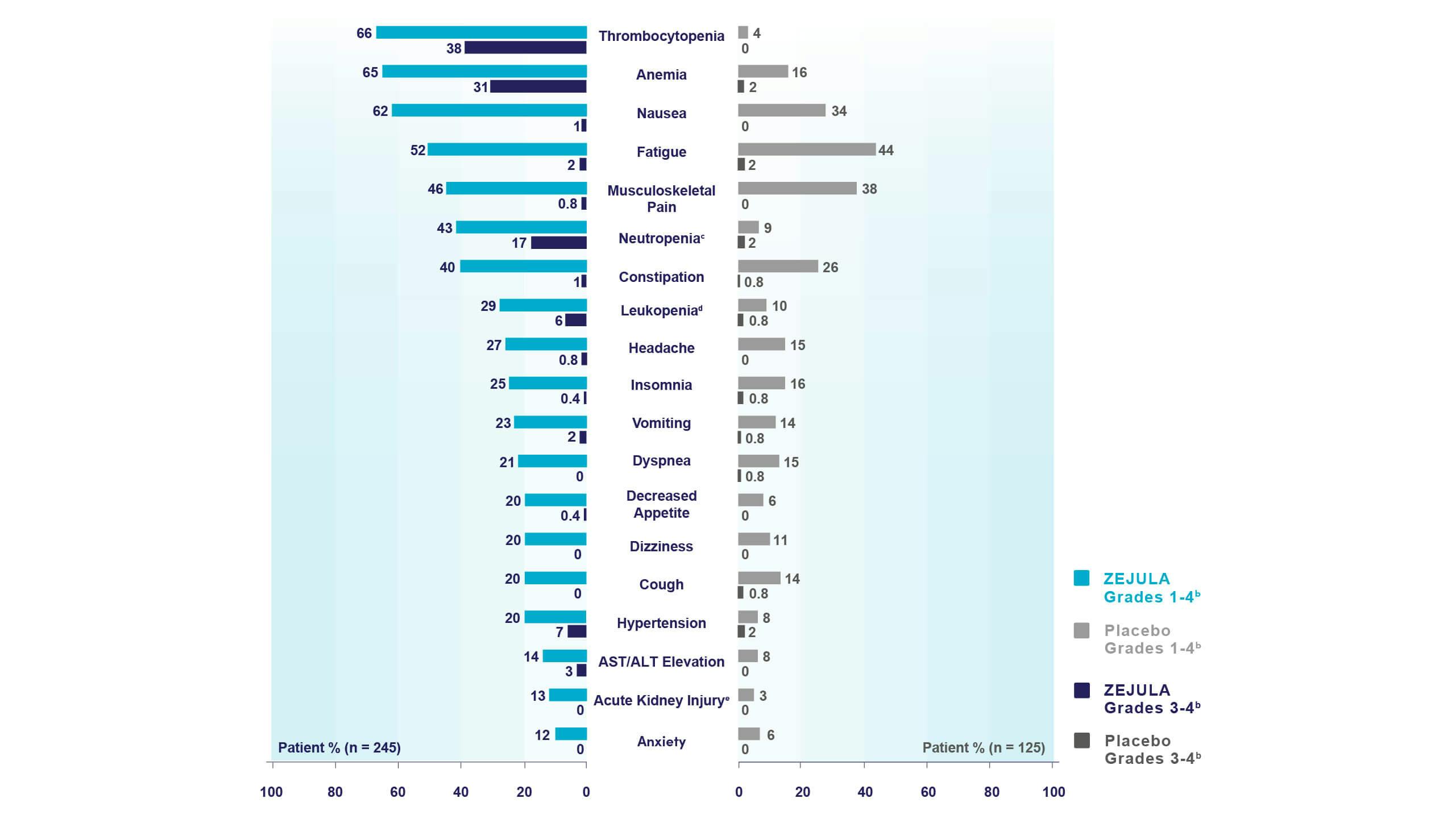
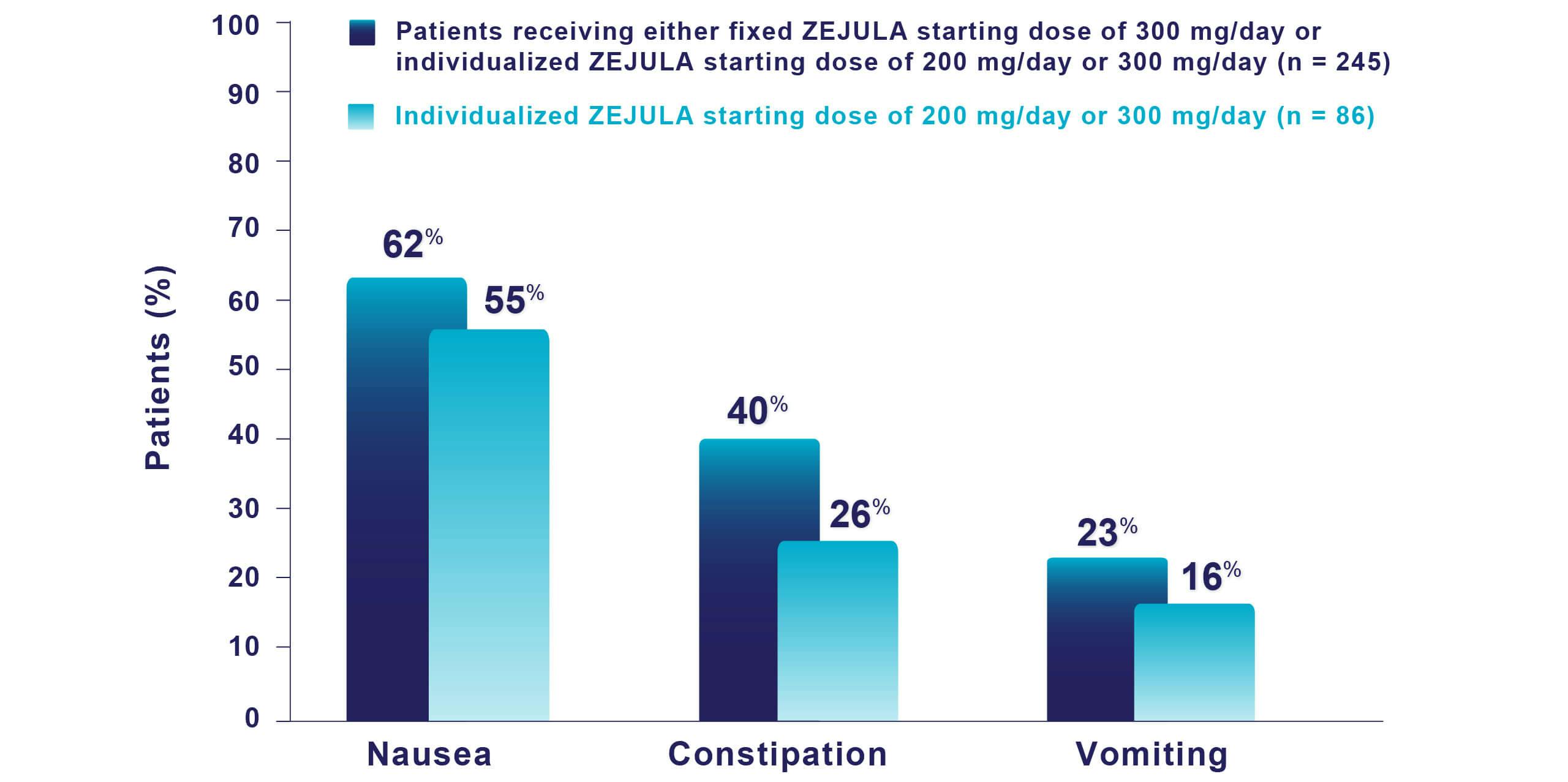
Most common adverse reactions (Grades 1-4) in ≥10% of all patients who received ZEJULA in PRIMA were thrombocytopenia (66%), anemia (65%), nausea (62%), fatigue (52%), musculoskeletal pain (46%), neutropenia (43%), constipation (40%), leukopenia (29%), headache (27%), insomnia (25%), vomiting (23%), dyspnea (21%), decreased appetite (20%), dizziness (20%), cough (20%), hypertension (20%), AST/ALT elevation (14%), acute kidney injury (13%), and anxiety (12%).
Common lab abnormalities (Grades 1-4) in ≥25% of all patients who received ZEJULA in PRIMA included: decreased hemoglobin (85%), decreased leukocytes (72%), decreased platelets (71%), decreased neutrophils (64%), increased glucose (62%), decreased lymphocytes (55%), increased alkaline phosphatase (48%), increased creatinine (40%), decreased magnesium (39%), increased AST (35%), increased ALT (32%), and increased calcium (31%).
Please see the full Prescribing Information for ZEJULA.